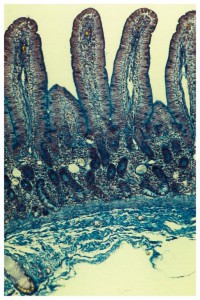
The Across Barriers Gastrointestinal models were calibrated with the 19 substances recommended by the FDA. With our Gastrointestinal Absorption Models the following questions could be answered:
-
How can new drugs be classified according to their gastrointestinal permeation or penetration capacity?
-
How can formulations be optimized?
-
How can substances be classified according to the BCS-guidelines of the FDA and EMA?
-
How to investigate the gastrointestinal bioadhesion capacity of different formulations?
-
How to investigate the transport mechanisms (e.g. BCRP, MRP2, pepT – 1, etc.)?
Models |
Assays |
|
Caco-2 |
|
|
Excised porcine gut (Ussing chamber) |
|